Abstract
Background: Relapse remains a serious event and main obstacle to permanent cure in childhood acute myeloid leukemia (AML). Reduction of measurable/minimal residual disease (MRD) assessed by real-time quantitative PCR (qPCR) during therapy is predictive of outcome in adults but persistent MRD positivity may be observed despite long-term remission and hamper accurate risk assignment. Sequential MRD determinations, rather than analysis at single landmark time points, may better capture the dynamics of leukemia eradication and identify relapse at molecular levels when applied in the post-therapy setting. We investigated the post-induction qPCR MRD kinetics in peripheral blood (PB) and bone marrow (BM) in a large cohort of childhood AML patients and demonstrate the utility of serial assessments for disease surveillance after therapy completion.
Methods: We collected the results from qPCR MRD analyses of 774 samples (BM 347, PB 427) from 75 children with AML harboring recurrent fusion transcripts (32 RUNX1-RUNX1T1, 24 CBFB-MYH11, 16 KMT2A-MLLT3 and 3 KMT2A-ELL). Patients were treated according to The Nordic Society for Paediatric Haematology and Oncology (NOPHO)-AML 2004 or NOPHO-DBH AML 2012 protocols (2004 - 2016), or AML-BFM 2012 protocol (2014 - 2016). Only patients who achieved complete remission (CR) during induction therapy and received standard-risk consolidation without allografting were eligible for the study.
Risk of relapse as a function of time of MRD positivity in sequential samples from BM or PB during consolidation therapy was modeled using restricted cubic splines. Considering shifting from MRD negative to positive in PB and MRD increments in BM above 5x10-4 as time-dependent variables, the Mantel-Byar method was applied to evaluate the prognostic impact of MRD kinetics during follow-up.
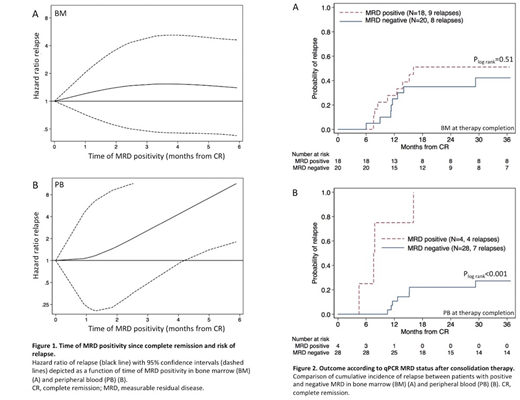
Results: Risk of relapse was independent of MRD persistence in BM during therapy, but showed a strong correlation with time of MRD positivity in PB where 8/8 patients with detectable MRD after first consolidation course relapsed (Figure 1A and 1B).
At therapy completion, MRD level in BM did not correlate to outcome, HR=0.64/MRD log reduction, 95% confidence interval (CI):0.32 - 1.26 (P=0.19), and there was no difference in 3-year cumulative incidence of relapse (CIR) according to MRD status (P=0.51, Figure 2A). Four patients remained MRD positive throughout consolidation therapy and all subsequently relapsed, whereas 7/28 patients who were MRD negative in PB at the end of therapy suffered relapse, 3-year CIR=27%, CI:14% - 49% (P<0.001, Figure 2B).
Shifting from negative to positive in PB during follow-up predicted subsequent relapse in 10/10 patients. All 253 PB samples collected during follow-up from 36 patients in continuous CR were MRD negative. In core binding factor (CBF) AML, persistent low-level MRD positivity in BM was frequent (detected in 38% of patients tested within six months of therapy completion) but an increment to above 5x10-4 heralded subsequent relapse in 12/12 patients. Pre-relapse MRD kinetics were delineated in 16 patients and revealed a median log increment/30 days of 0.8 (range:0.4 - 1.5) in CBF patients versus 2.2 (range:1.1 - 3.7) in patients with KMT2A-MLLT3 (P=0.008).
Perspectives: This study demonstrates that qPCR MRD monitoring in PB, rather than BM, represents an accurate discriminator of prognosis and is a highly informative tool for disease surveillance in childhood AML. Early relapse detection during follow-up may facilitate preemptive therapy strategies of molecular relapse, possibly improving relapse treatment outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal